Powerful Waste & Inventory Management for Biotech Facilities
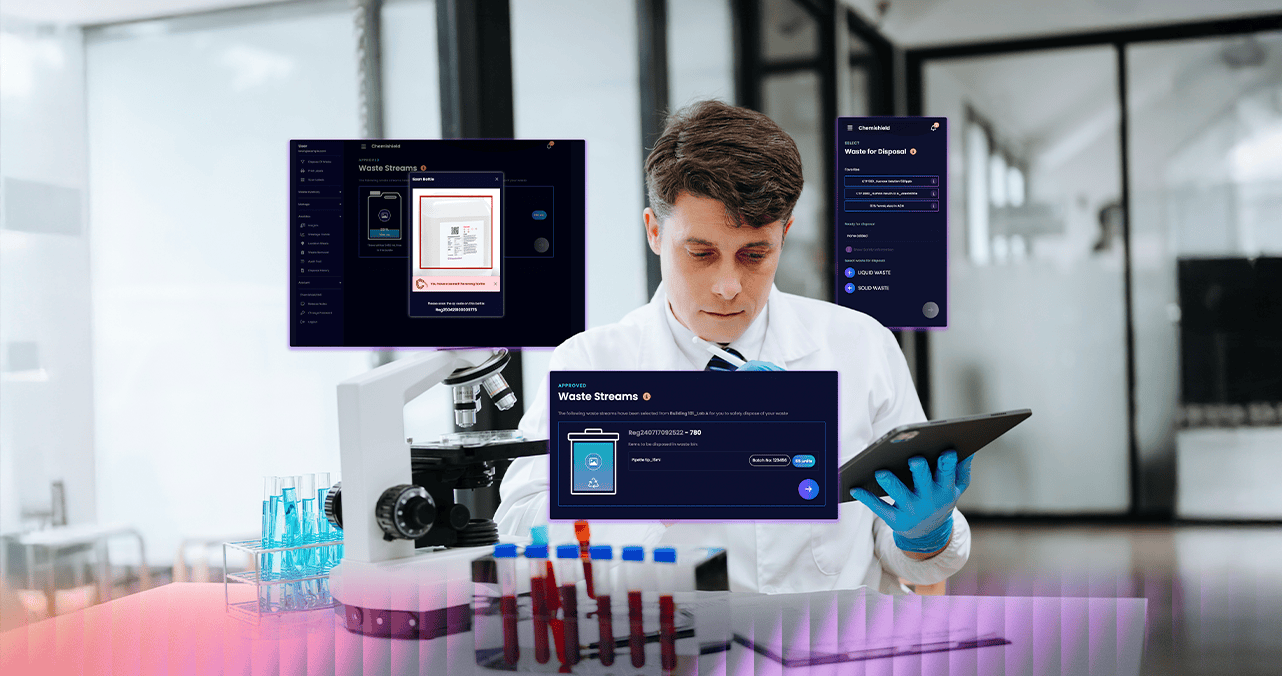
Accelerate Lab Safety and Efficiency with Advanced Biotech Waste & Inventory Management Software
Biotech environments demand rigorous control over hazardous waste and high-value inventory. Chemishield is designed to meet the precision, traceability, and compliance standards your facility requires—whether you're managing lab waste, chemical reagents, or temperature-sensitive biological materials.
From R&D to production, Chemishield empowers biotech teams to streamline waste handling, maintain full compliance, and manage critical lab inventory with visual precision through our intelligent picture-referenced IMS system.
The Biotech Waste & Inventory Challenge
Frequent generation of hazardous biological and chemical waste
High-value, sensitive inventory with limited shelf life
Strict biosafety and environmental regulations (e.g., BSL, CLP, GxP)
Risk of cross-contamination or mismanagement due to manual processes

Built for the Precision of Biotech Environments
How Chemishield Solves These Challenges
Chemishield is purpose-built for highly regulated environments. Our platform offers modules for both waste and inventory management—ideal for cleanrooms, wet labs, and production facilities.

What You Can Expect
Chemishield supports biotech organizations with compliant, and intelligent waste and inventory management. Here’s how:
Enhanced Compliance
Assurance Meet and exceed biotech-specific regulations such as GxP, CLP, and local biosafety standards. Chemishield creates a fully traceable audit trail—automatically.

Protect Research Integrity
Prevent material degradation and loss with real-time inventory monitoring, expiry alerts, and our visual reference system that eliminates guesswork and improves stock accuracy.
Boost Operational Efficiency
Digitize routine waste handling and inventory processes. Automate reporting, ensure real-time data access, and reduce reliance on outdated spreadsheets and manual logs.
Support for Sustainability and ESG Goals
Optimize usage, prevent unnecessary procurement, and reduce the environmental impact of lab waste. Chemishield provides the insights you need to align with corporate sustainability initiatives and reporting standards.
See The Results

The Success of Chemishield at a Leading Biotech Facility
This biotech research facility is a key player in the development of advanced biologics and gene therapies, with operations spanning early-stage research to clinical trial manufacturing.
June 26, 2025
Frequently Asked Questions
1. What is a laboratory inventory system?
A laboratory inventory system is a digital solution that tracks and manages all materials used within a lab environment, including reagents, chemicals, biological samples, consumables, and equipment. In biotech, where precision, compliance, and repeatability are paramount, having a robust inventory system is essential to ensure experimental integrity and avoid regulatory issues.
Chemishield offers a purpose-built laboratory inventory system for biotech labs, enabling real-time tracking of stock levels, expiry dates, storage locations, and usage history. It eliminates manual spreadsheets, supports chain-of-custody for sensitive materials, and integrates seamlessly with waste management, ensuring a fully traceable, compliant workflow across R&D, QA/QC, and production environments.
2. How to manage laboratory inventory?
Managing biotech lab inventory requires a structured, data-driven process:
- Register new stock upon receipt with complete metadata (batch, expiry, storage conditions).
- Track usage in real-time, linking materials to projects or protocols.
- Set thresholds and expiry alerts to avoid stockouts or wastage.
- Control access to sensitive or regulated substances.
- Audit inventory regularly to meet GxP, ISO, and safety standards.
- Link inventory to waste outputs for accurate reporting and ESG tracking.
Chemishield automates this process from end to end. Our platform ensures accurate, compliant inventory control while reducing administrative burden. It supports multi-location labs, access control, and full audit trails, helping biotech companies maintain operational efficiency, traceability, and regulatory confidence.
3. What are the four types of inventory management?
In biotech labs, inventory is often managed through a combination of these four models:
- Perpetual Inventory – Real-time tracking with automatic updates.
- Periodic Inventory – Manual counts performed at scheduled intervals.
- Just-in-Time (JIT) – Stock is ordered only when needed, reducing storage costs.
- ABC Analysis – Materials are categorised by value and usage rate (e.g., high-value reagents vs. general consumables).
Chemishield supports all four inventory management models, allowing biotech organisations to configure the system to suit their operational style. Whether you're running a high-throughput screening lab or a tightly controlled clinical development facility, Chemishield offers the visibility, control, and flexibility to optimize lab operations.
4. What is the main objective of an inventory management program in a laboratory?
The primary goal of inventory management in a biotech lab is to ensure materials are available, traceable, and compliant, while minimising waste, cost, and regulatory risk. This is critical for reproducibility, safety, and alignment with GxP, ISO 17025, and environmental standards.
Chemishield delivers strategic value by uniting inventory control with compliance and sustainability. Our system reduces expired stock, supports data-driven procurement, links inventory to waste output, and ensures labs are always audit-ready. It transforms inventory from a cost centre into a strategic enabler of quality, innovation, and ESG performance.


