Intelligent Waste & Inventory Control for Pharmaceutical Facilities
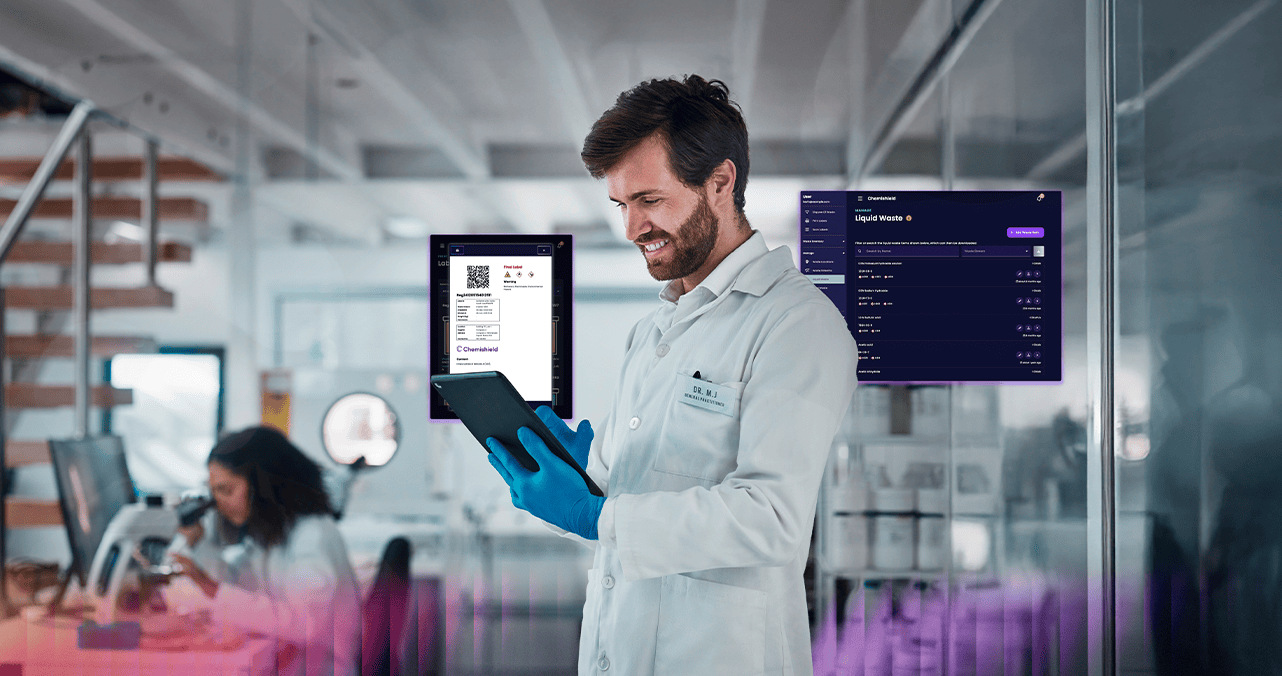
Master Regulatory Complexity with Smart Waste Management
Pharmaceutical environments demand airtight compliance, precise waste categorization, and full traceability. Chemishield is purpose-built for GMP-regulated settings, enabling pharma companies to:
• Track hazardous and non-hazardous waste from generation to disposal
• Automate documentation for audits and regulatory reporting
• Manage inventory across multiple locations with real-time visibility
From controlled substances to flammable liquids, Chemishield helps you stay compliant with strict industry standards like GMP, FDA 21 CFR Part 11, and EMA regulations.
Common Pain Points in Pharmaceutical Waste Management:
Strict regulatory oversight (FDA, EMA, HPRA)
Complex waste classification and labelling requirements
Fragmented systems across labs, cleanrooms, and production
Time-consuming audits and compliance reporting

Trusted by Pharma Teams for Lab-to-Plant Efficiency
How Chemishield Solves These Challenges
Chemishield simplifies waste and inventory control from research to production. Here’s how we help pharmaceutical companies transform compliance into a competitive advantage:

What You Can Expect
Chemishield empowers pharmaceutical facilities to operate with greater control, safety, and sustainability. Here’s how:
Regulatory Confidence
Instantly generate compliant documentation, digital signatures, and a validated audit trail for inspections.
Operational Control
Reduce manual handling, streamline categorization, and unify workflows across R&D, QA/QC, and production teams.
Sustainability Insights
Cut waste, control overstocking, and report clearly on sustainability targets as part of your ESG commitments.
See The Results

The Success of Chemishield at Pfizer Grange Castle
Our client is an American multinational pharmaceutical company headquartered in New York City. They develop and produce medicines and vaccines for immunology, oncology, cardiology, endocrinology, and neurology treatment.
June 26, 2025
Frequently Asked Questions
1. What are the methods of pharmaceutical waste management?
Pharmaceutical waste can be managed through several methods, including high-temperature incineration, chemical neutralisation, encapsulation, autoclaving (for certain materials), and secure landfilling. While these are the final treatment options, proper waste management begins at the source, with accurate classification, segregation, and documentation.
Chemishield transforms traditional pharmaceutical waste management by digitising the process from start to finish. Our platform enables labs, manufacturers, and hospitals to correctly identify and track waste items from the moment they're generated, ensuring they’re directed to the appropriate disposal route in full compliance with regulations. By removing paper-based systems and guesswork, Chemishield improves efficiency, reduces errors, and ensures sustainable, auditable outcomes.
2. How do you classify pharmaceutical waste?
Pharmaceutical waste classification involves identifying the type of waste based on its chemical properties, toxicity, flammability, reactivity, and regulatory categorisation (e.g., cytotoxic, hazardous, non-hazardous, controlled substances). Misclassification can lead to compliance failures, safety hazards, and inflated disposal costs.
Chemishield brings expert-level accuracy to this complex process. With built-in logic, the system guides users to correctly categorise waste every time, whether it’s a cytotoxic drug, a flammable solvent, or a general expired product. This automation reduces training needs, human error, and audit exposure, making regulatory compliance far easier for any organisation handling pharmaceutical waste.
3. What are the problems associated with pharmaceutical waste?
Pharmaceutical waste presents several problems: environmental contamination, health and safety risks to staff, complex disposal regulations, poor traceability, and excessive costs due to improper segregation or over-disposal. Manual processes and inconsistent practices only exacerbate these issues.
Chemishield directly solves these challenges by offering a controlled, digital environment for waste management. Our platform enforces correct classification and segregation at the point of generation, tracks every waste item through to disposal, and generates real-time compliance reports. The result is safer workspaces, improved environmental outcomes, and reduced financial and regulatory risk.
4. What is the correct method of disposal for waste pharmaceuticals?
The correct disposal method depends on the specific pharmaceutical waste type. For example, cytotoxic drugs typically require high-temperature incineration, while certain non-hazardous materials may be eligible for landfilling or neutralisation. Regulatory compliance is non-negotiable, and errors in classification or routing can result in severe penalties.
Chemishield ensures every pharmaceutical waste item follows the correct disposal path. Our system automates classification and links it to the appropriate disposal method, generating labels, documentation, and collection instructions with minimal staff input. This removes ambiguity, strengthens compliance, and protects your organisation from risk.
5. How is waste treated in the pharmaceutical industry?
In the pharmaceutical industry, waste is typically treated through incineration, chemical treatment, or encapsulation, depending on its hazard class. The treatment process is heavily regulated and audited, requiring accurate documentation and consistent classification before it ever reaches a treatment facility.
Chemishield supports the pharmaceutical industry by standardising waste management across facilities. Whether in R&D, manufacturing, or QA/QC, our platform enforces best practices and ensures traceability at every stage. By integrating Chemishield, pharmaceutical companies can scale operations while maintaining full environmental and regulatory compliance, and demonstrating ESG leadership.
6. What are the two different types of pharmaceutical waste and pharmaceutical waste containers?
Pharmaceutical waste is typically divided into two main categories:
- Hazardous waste: Includes cytotoxic/cytostatic drugs, flammable solvents, or reactive compounds.
- Non-hazardous waste: Includes expired over-the-counter medicines, saline solutions, or packaging waste.
Each type must be stored in specific, compliant containers e.g., purple bins for cytotoxic waste, yellow bins for clinical waste, or black bins for general non-hazardous waste, each clearly labelled with relevant hazard and regulatory information.
Chemishield eliminates the guesswork in this process. Our platform tells users exactly which container to use, automatically generates compliant labels, and ensures all waste is traceable to its point of origin. This drastically reduces segregation errors, improves safety, and increases efficiency in pharmaceutical waste handling.


